My favorite way to relax at the end of a long day is to sit in a comfy chair with a good book and a glass of Amaretto Disaronno. That little glass (not sure if that size has a name...) holds about 100cc and I can nurse it for about 20 sips, each just enough to coat the tongue to savor that intense almond flavor for a long time while reading a couple chapters.
For a while I was chilling my amaretto with an ice cube, but that of course diluted the drink as it melted, so yesterday I experimented with a trial of amaretto ice cubes. Didn't work. Perhaps not evident in the snapshot above is that the amaretto in the final two tray slots did not expand and freeze - it just converted into a cold slush which I had to spoon into the glass.
Searching the web, I found an excellent article at The Spruce Eats with all the relevant information. Amaretto is 64 proof, with a freezing point of -23C (-10F) and is "safe for the freezer" (meaning that it will chill but not freeze).
I might try this project again next winter. It's not unusual to get nighttime temps of -20F or lower in south central Wisconsin in midwinter, so I may set out an ice cube tray with amaretto then.
I'll finish this post by appending information from a post I wrote many years ago about cold distillation. I never have found confirmation or refutation of the legend of Napoleon's frozen wines during his retreat from Russia.
"Freezing Wine" (posted in 2009)
Where we live in the Upper Midwest it is a common practice for people to use their screen porch to chill and even store foods. Several days ago we left a half-full bottle of wine there and discovered that it had frozen solid; temperatures here have been reaching -15 F (-26 C) at night.
Since this was a fine ($8) Wisconsin wine (Prairie Fume, Wollersheim - #5 of the top 100 wines east of the Rockies in 2004), we decided to thaw it and finish the bottle. Imagine my puzzlement when, upon thawing, the wine bottle exhibited a fairly substantial precipitate of crystals on the bottom. After receiving reassurances from my wife that she had not slipped any surprises into the wine, I tried to decant the supernatant, but the crystals spilled into the glass and I drank them. The flavor was o.k. and I appear to have suffered no ill effects.
Googling this subject today, I found the identity of the crystals:
Chances are that freezing and thawing won't seriously damage the wine itself, although on general principles I wouldn't try it with a treasured rarity. Near-freezing temperatures may precipitate out some of the wine's natural acidity in the form of insoluble tartrate crystals, but most authorities argue that this doesn't perceptibly affect the flavor of the wine.Tangentially related is "ice wine" -
"... a type of dessert wine produced from grapes that have been frozen while still on the vine. The sugars and other dissolved solids do not freeze, but the water does, so the result is a concentrated, often very sweet wine. The most famous (and expensive) ice wines are German Eiswein and Canadian ice wine..."There were a number of discussion threads in which people wrongly proclaimed that freezing wine was impossible because of the alcohol content. The correct response is that the freezing point of pure alcohol is -173 F, but wine with low alcohol content can freeze with temps in the low teens Fahrenheit.
Which serves as an introduction to my next query. I seem to remember reading somewhere that during Napoleon's winter campaign to Russia the wine froze (as of course did the horses and the soldiers), and that from the frozen wine bottles a purer alcohol could be harvested. Googling that question brought me to this comment at a "home brew" thread:
"Grandfather would make seasonal wines. Whatever fruit was in season... He then had a rather unique way to turn the wine into brandy (cognac). He would transfer the wine to a plastic jug and put it in the freezer. The water in the wine would freeze, but the alcohol would not. He then transferred this to bottles and capped them. The result being that we had natural flavored brandy of all types..."What's being described there seems to be a form of cold distillation. That led me to a very interesting site entitled "Alcoholic Drinks of the Middle Ages."
Another method, known as fractional crystalization, is done by inverting the process and freezing the beverage instead of boiling it. This works for very similar reasons to that of normal heat distillation, namely, the differential in freezing points of the two liquids involved. Water freezes at a temperature of 0 C, while ethyl alcohol does not freeze until reaching -114 C. This allows the water to be frozen out of the liquid, leaving behind the ethyl alcohol, as well as the other alcohols and esters. This produces a drink of a rather different character from heat distillation, as it contains everything except water, while heat distilled beverages leave everything behind except alcohol.The website also has a nice table of freezing points for different alcohol concentrations, explanations of the differences between brandies, whiskies, and cognacs, and an introduction to the distilling of liquor in medieval times, citing recipes in "Delightes for Ladies" -
"...a book of recipes and household hints for women, written... in 1602. Its full title is Delightes for ladies: to adorn their persons, tables, closets, and distillatories with beauties, banquets, perfumes and waters. A successful book in its day, some of the recipes have survived to be in relatively common use even 400 years later, in particular the various mixed alcoholic beverages."Ladies of the 1600s had... "distillatories" ??
At this point I'll have to stop exploring the subject because I'm getting way out of my depth. Perhaps Morchava will know of something in her library of medieval literature, or maybe Gail over at Scribal Terror can come up with more information or images of ladies' distillatories.
And all this because we left a bottle of wine on the screen porch overnight...
Addendum: here's an illustration of a medieval still that Gail found -

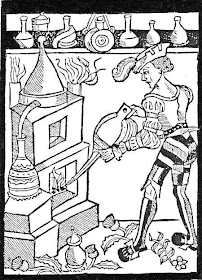
The distilleries can be used for making essential oils, very useful in perfume, especially if you live in a culture without hot running water or soap.
ReplyDeleteCame down to say this same thing. Much medieval-inspired fiction and fantasy will have reference for the lady-of-the-manor's stillroom, where she would produce essential oils for perfume, cooking, or medical uses.
DeleteDistillatory is what "still" is short for.
ReplyDeleteThere's a good illustration of a medieval/renaissance still here:
ReplyDeletehttp://www.hypatiamaze.org/isaac/alchemist.jpeg
Interesting post...however the "home brew" forum is located in the bowels of White Supremacist network...
ReplyDeletearchive location of "Alcoholic Drinks of the Middle Ages":
ReplyDeletehttps://web.archive.org/web/20111203205742/http://mysite.verizon.net/~mshapiro_42/cspirits.html
I-)
So didn't you get a simple answer to your initial reason for making Amaretto cubes to chill your drink? Just put the whole bottle in your freezer and serve it super cold. You know it won't freeze.
ReplyDeletehah, finally a topic again where I can add my 0.02$.
ReplyDeleteThere is a mention of German icewine. 2019 was the first year that not a single drop of icewine was put into the cellars, 2020 I think the prognosis is that no vineyard will even try (2018, about 600 hectares in the biggest wine growing region were designated for icewine, 2019, only 42 were declared).
The issue is that you need below -7 Celsius, which does not only not happen early enough in the winter, but rather not at all anymore. What also doesn't help is that the summers are hotter, so the grapes are ripe earlier, which also makes the possible window for harvest shorter in winter, since the grapes will spoil.
And my second 0.02$: Freezing and removing the frozen water is also used to make super-strong beers. The current record sits at a whopping 57% of alcohol content. I have had some of the ~ 20% stuff, didn't like it very much: http://www.schorschbraeu.de/
Try whisky stones, there are different types made of stone or stainless steel. They hold the coldness, but have no chance of adding water to dilute the drink.
ReplyDeleteFreeze distillation is still a (somewhat) common practice to make homemade applejack and other random booze. You can find plenty of folks talking about this method on the "PrisonHooch" sub-reddit (because of course there's a sub-reddit for it):
ReplyDeletehttps://www.reddit.com/r/prisonhooch/search?q=freeze&restrict_sr=1
In Sweden, cold akvavit, a caraway-flavoured spirit, usually accompanies herring when eaten on a holiday, such as Midsummer's Eve. Everyone I know just puts the whole bottle in the freezer for a few hours to produce a really cold drink. The cold improves the taste somewhat (but not much, imo).
ReplyDeletehttps://en.wikipedia.org/wiki/Akvavit
Freeze-distilled mare's milk may have been the first distilled beverage -
ReplyDeletehttp://www.wondersandmarvels.com/2013/05/drunk-on-horse-milk-fermented-koumiss.html
and how did you miss a website named wonders and marvels?
The 24-hour length of a day places some constraints on me.
Delete